What are some common quality criteria used to measure URS completeness in 2024?
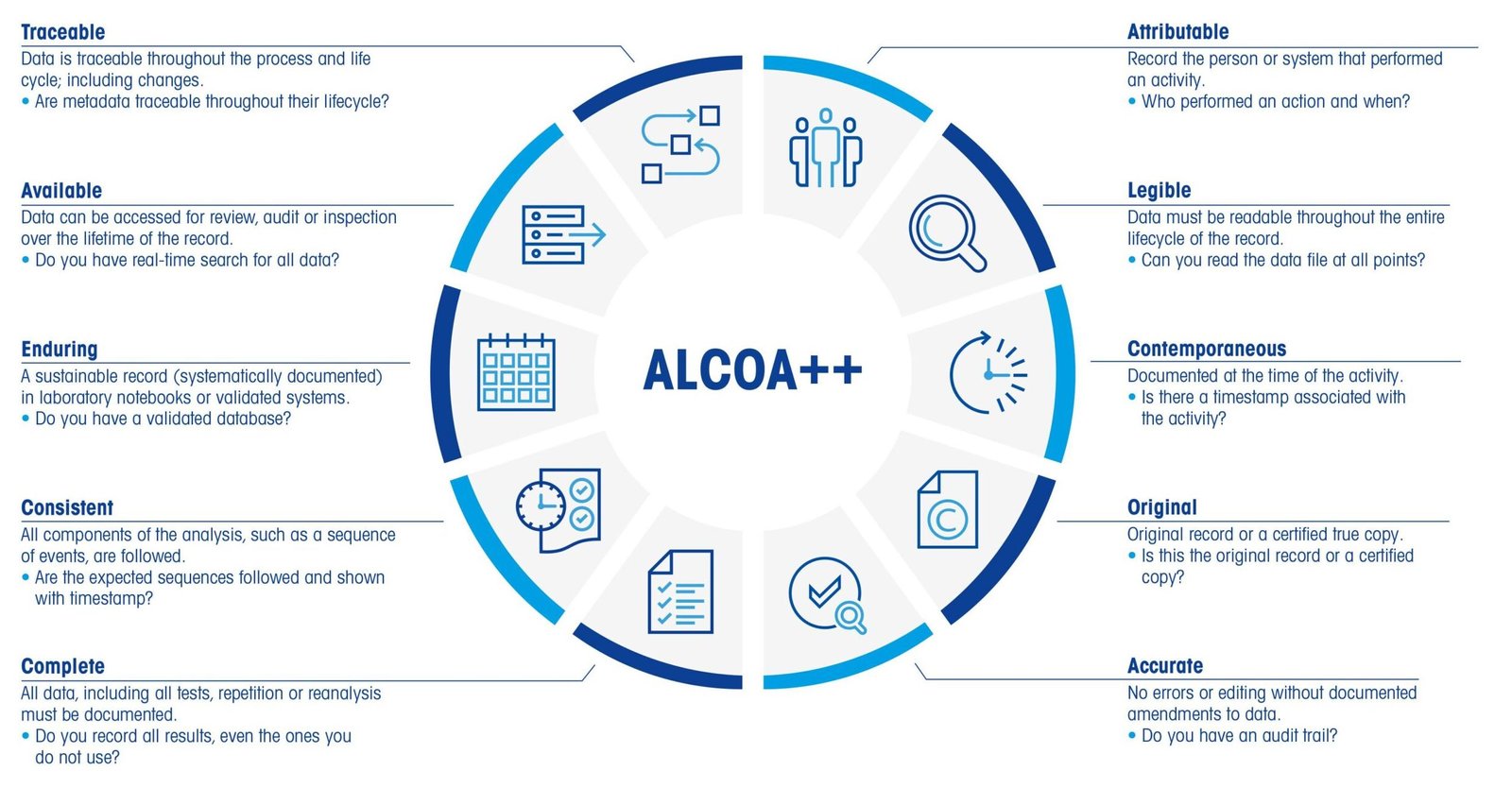
To ensure the completeness and quality of a User Requirements Specification (URS), several common quality criteria can be employed. These criteria help assess whether the URS effectively captures all necessary user needs and expectations, providing a solid foundation for subsequent phases of development and validation. 1. Clarity of URS Definition Clarity refers to the unambiguous … Read more