Understanding ALCOA Principles in Pharmaceutical Data Integrity Key to FDA Compliance 2024
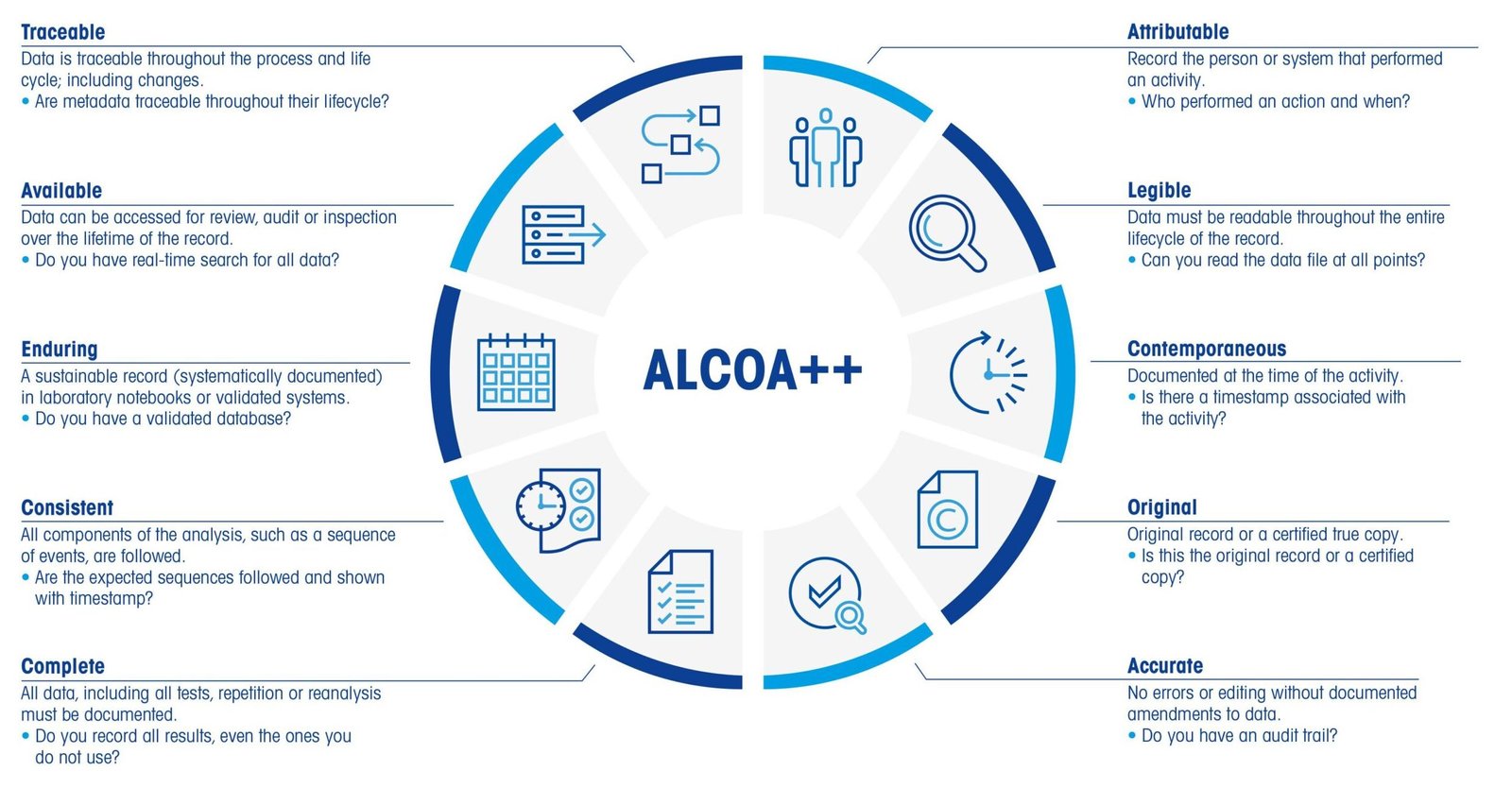
ALCOA is a set of principles (Alcoa Principles) that ensure the integrity of data, particularly important in the life sciences sector. It’s used by the FDA (Food and Drug Administration) and is becoming increasingly crucial for Good Manufacturing Practices (GMP). In the realm of pharmaceuticals, maintaining accurate and reliable data is not just a regulatory … Read more